
So, to convert mmH2O to mmHg, all we have to do is Multiply the number by 0.073556127270818.
#1000 mmhg to atm how to#
How to convert mmH2O to mmHg (mmH2O to mmHg) If you use our mmH2O to mmHg conversion tool, you will know that one mmH2O is equal to 0.073556127270818 mmHg. What is the conversion factor from mm H2O to mm Hg? The pressure in pounds per square inch is equal to the atmospheres multiplied by 14.695964. To convert an atmospheric measurement to a pounds per square inch measurement, multiply the pressure by the conversion ratio. What unit of pressure relates atmosphere to PSI? This means you should convert mmHg to atm Multiply your number by 0.0013157896611399. One millimeter of mercury is equal to 0.0013157896611399 atmospheres. How to convert atmospheric pressure from ATM to mmHg?Ītmosphere (atm) is a unit commonly used to express the average atmospheric pressure at sea level. Or, a 1-foot column of water weighs 1 square inch square. Translated, this means that a column of water that is 1 square inch and 2.31 feet high weighs 1 pound. The relationship between PSI and foot of the head is the 2.31 feet head = 1 PSI. This measurement is a comparison between the barometric pressure and the pressure inside the vacuum. It is used as a measurement of “ inches of mercury”, which translates to millimeters of mercury for the metric system. Put simply, Hg is the unit of measurement for the work output of a specific vacuum.
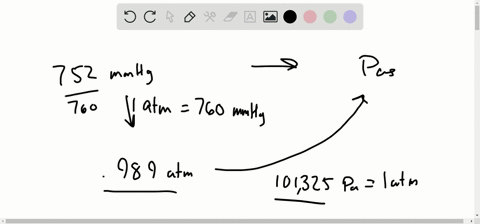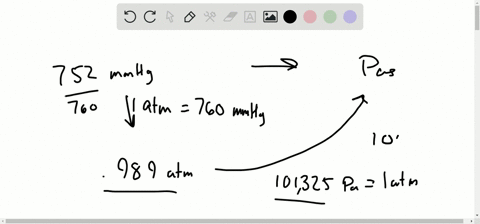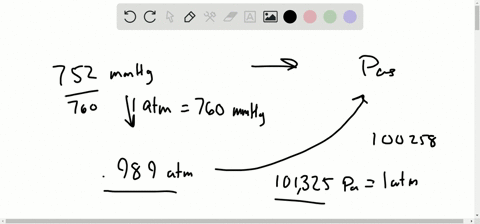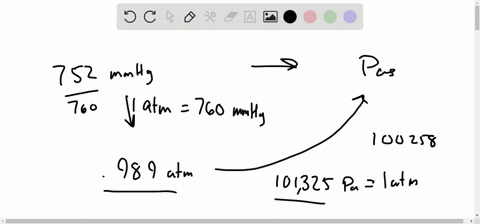

Let’s see some interconversion from 1 atm. Note: We know the interconversion between the units to do various calculation problems. So we can expresses the equation of pressure as, We know that pressure is the force exerted in a specific area and it is calculated by dividing the force applied by the area on which the force was applied. So before going into the solution part of the question, let’s discuss some concepts about pressure and its unit.

In the question, it is asked that we have to convert the given value of unit in mm Hg to atmospheres of pressure.The question is simply about te unit conversion of values between the units of pressure, We should be familiar with 1 atm value equal to how much value of mmHg. Hint: The approach for the question should be done by focusing on the unit conversion of the value from mmHg to atmospheres of pressure in atm.


 0 kommentar(er)
0 kommentar(er)
